In a little more than one month, from March 19 to May 1, the Mechanical Ventilator Milano (MVM) has gone from conception to reality, as it is now shifting to production and to support of patients affected severely by COVID-19. On May 1, 2020, the United States Food and Drug Administration (U.S. FDA) declared that the MVM falls within the scope of the Emergency Use Authorization (EUA) for ventilators.
The MVM is an innovative ventilator, conceived and designed by an international collaboration of particle physicists and developed in cooperation with other relevant scientific communities. Its mechanical design is simple, using a small number of parts to facilitate rapid production. The powerful and complex control unit, programmed by a large number of researchers, results in strong and safe performance for the care and recovery of COVID-19 patients. Achieving this result in such a short time was made possible thanks to the cooperation of laboratories, institutes, universities and companies mainly across Italy, Canada, and the United States, maximizing the benefits that come from the sharing of skills and resources.
The MVM challenge
A fraction of the people infected with COVID-19 can become severely ill, needing help to breathe. This has created a worldwide demand for ventilators. To address this critical global issue, the MVM collaboration took on the challenge to design, develop, build, and certify a safe and powerful, ventilator. A very important feature of the MVM is the simplicity of its mechanical design, which allows for quick production. Another important feature is the sophisticated control system, which makes available the two ventilation modalities required for the care of COVID-19 patients, while also ensuring ease of use for medical personnel.
The MVM initiative originated in the framework of the GADM Global Argon Dark Matter Collaboration, an international scientific collaboration engaged in the search of dark matter with experiments at Istituto Nazionale di Fisica Nucleare’s Gran Sasso Laboratory in Italy and SNOLAB in Canada. This research involves gas handling systems and complex control systems, the same technologies required in mechanical ventilators.
While in lockdown for the COVID-19 pandemic in Milan, Italy, Cristiano Galbiati (Gran Sasso Science Institute, INFN, and Princeton University), the spokesperson for the GADM Collaboration, recognized the need for additional ventilators early in the pandemic. He launched the MVM project and started the development of a first prototype. With support from INFN Italy’s National Institute for Nuclear Physics; groups from the Universities of Bergamo, Brescia, GSSI Gran Sasso Science Institute, Insubria, L’Aquila, Milano Bicocca, Milano “La Statale”, Napoli “Federico II”, Pisa, Pavia, Roma “La Sapienza”, Siena; CNR National Research Council; Istituto Superiore di Sanità; Azienda Ospedaliera San Gerardo of Monza; and Elemaster, project leader and coordinator of the other companies involved AZ Pneumatica, Saturn Magnetic, Bel Power Europe, Nuclear Instruments, CAEN and Camozzi, the MVM collaboration produced an initial prototype, which fully demonstrated the viability of the conceptual design.
The laboratory facility for the development of the first units was made available by Elemaster S.p.A of Lomagna (LC), Italy. In addition to creating the MVM controller printed circuit board in-house, Elemaster also led the assembly and prototype testing in collaboration with the partner companies involved. The Elemaster International Design Center, as MVM design authority, led the submission to the U.S. FDA. laboratory facility for the development of the fi
The collaboration quickly expanded to include three national laboratories in Canada, including Canadian Nuclear Laboratories (CNL), TRIUMF and SNOLAB, through the leadership of Nobel laureate, Dr. Arthur McDonald of Queen’s University.
The US collaboration includes people from Fermi National Accelerator Laboratory (Fermilab) and the Princeton Plasma Physics Laboratory, two of the Department of Energy’s national laboratories as well as staff from several US universities.
The European collaboration also includes researchers from Politecnico di Milano and Museo della Fisica e Centro Studi e Ricerche Enrico Fermi of Italy; APC, SUBATECH and Mines Paris Tech of France; CIEMAT and LSC, CAPA-UZ and ARAD of Spain; AstroCeNT (CAMK PAN) of Poland; MPA Garching of Germany; University of Toronto of Canada; Rochester University, University of California Los Angeles, University of Houston, University of Massachussets at Amherst, University of Nebraska-Lincoln of the United Stated; Liverpool University and University of Oxford of the United Kingdom.
Getting the MVM ventilator to patients requires collaboration beyond nuclear and particle physicists. Government departments, regulators, manufacturers and health care providers have made valuable contributions to the project.
Clinicians sited in Italy, Canada, and in the United States provided guidance to ensure medical considerations were properly integrated into the design. Anesthesiologists from the COVID-19 wards in Lombardy, one of the districts most severely hit by the pandemics, played a special role in providing detailed guidance for the design of the unit. Detailed testing and qualification of performance were carried out at Ospedale San Gerardo in Monza, Italy.
The MVM Collaboration is being enthusiastically supported by industry partners who are assessing parts availability, evaluating supply chains, and who will soon carry out the mass manufacturing. The laboratory facility for the development of the first units was made available by Elemaster S.p.A. of Lomagna (LC), Italy, which also took primary responsibility for the submission to the U.S. FDA.
Vexos Inc. will manufacture and distribute the MVM Ventilator under an exclusive license from Elemaster for the Americas and other territories. In order to support the demand of the MVM Ventilators, Vexos has formed a special task force team with key members of the engineering, quality, supply chain and manufacturing groups at their ISO 13485:2016 (Quality Management Systems for Medical Devices) accredited facilities in LaGrange, Ohio, USA and Markham, Ontario, Canada. Since March, Vexos has been preparing extra manufacturing capacity and an increased supply chain pipeline for components and materials to meet the expected high demand for MVM ventilators.
To facilitate rapid certification of the final design, additional direction is being provided by Health Canada, the US Air Force, the US FDA, the Italian “Ministero della Salute” (Ministry of Health), and the Italian “Istituto Superiore di Sanità”.
The MVM design
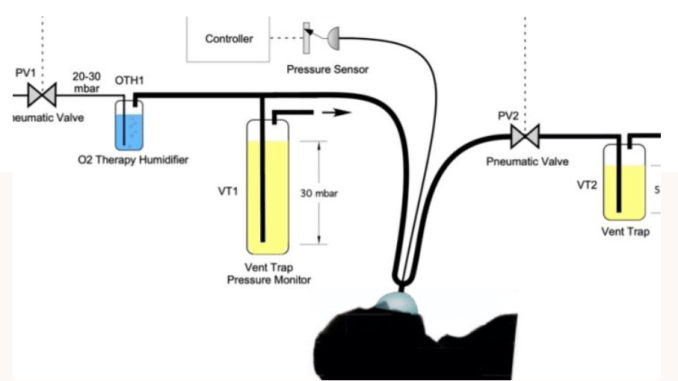
The MVM ventilator is inspired by the Manley ventilator, which was developed by Roger Manley in 1961, based on “the possibility of using the pressure of the gases from the anesthetic machine as the motive power for a simple apparatus to ventilate the lungs of the patients in the operating theatre”. The MVM is designed to similarly meet the requirements of a ventilator as simply as possible. The MVM also incorporates advanced features directly recommended by anesthesiologists participating who provided care for COVID-19 patients in Lombardy, the region in Italy most severely hit by the COVID-19 epidemics. The MVM features electrically driven pneumatic valves rather than mechanical switches and uses a stripped-down mechanical design. This enables fast progress from design to quick, inexpensive mass production of safe, reliable ventilators for hospitals and patients around the world. The modular design can also be adapted to swap out parts based on their availability in different regions of the world.
The final design of the MVM ventilator will soon be released on arXiv.org. It will be licensed under the CERN OHL v2.0 by the Fondazione Aria.
Fonte: https://rtsleepworld.com/2020/05/12/particle-physicists-develop-new-vent-for-covid-19-use/